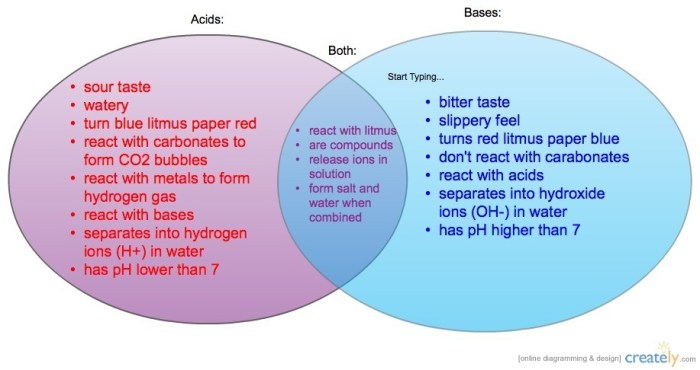
The Venn diagram for acids and bases provides a visual representation of the relationship between these two fundamental chemical concepts. Acids, defined as substances that donate protons (H+ ions), and bases, defined as substances that accept protons, exhibit distinct properties that can be effectively illustrated using this diagram.
The diagram is constructed with two overlapping circles, one representing acids and the other representing bases. The overlapping region, labeled “Arrhenius acids and bases,” encompasses substances that satisfy both the Arrhenius and Brønsted-Lowry definitions of acids and bases.
Venn Diagram of Acids and Bases: Venn Diagram For Acids And Bases
Introduction
A Venn diagram is a graphical representation of the relationship between two or more sets. It can be used to show the similarities and differences between the sets.
In this article, we will use a Venn diagram to represent the relationship between acids and bases. Acids and bases are two of the most important concepts in chemistry, and they play a role in many different chemical reactions.
Acids and Bases
The Brønsted-Lowry definition of acids and bases is that an acid is a substance that can donate a proton (H+), and a base is a substance that can accept a proton.
Strong acids and bases completely dissociate in water, while weak acids and bases only partially dissociate. The strength of an acid or base can be determined by measuring its pH.
Venn Diagram of Acids and Bases, Venn diagram for acids and bases
The Venn diagram below shows the relationship between acids and bases.

The overlapping region of the Venn diagram represents the set of substances that are both acids and bases. These substances are called amphoteric substances.
Arrhenius acids and bases are a subset of Brønsted-Lowry acids and bases. Arrhenius acids are substances that produce H+ ions in water, and Arrhenius bases are substances that produce OH- ions in water.
Examples of Acids and Bases
Some common examples of acids include hydrochloric acid, sulfuric acid, and nitric acid. Some common examples of bases include sodium hydroxide, potassium hydroxide, and calcium hydroxide.
The properties of acids and bases can be used to predict their behavior in chemical reactions. For example, acids react with bases to form salts and water.
Applications of Acids and Bases
Acids and bases have a wide range of applications in everyday life. They are used in industry, medicine, and agriculture.
For example, acids are used to make fertilizers, batteries, and plastics. Bases are used to make soaps, detergents, and paper.
Commonly Asked Questions
What is the purpose of a Venn diagram for acids and bases?
A Venn diagram for acids and bases is used to represent the relationship between these two types of substances, visually depicting their similarities and differences.
How do you determine the strength of an acid or base using a Venn diagram?
The strength of an acid or base is not directly represented in a Venn diagram. However, the diagram can be used to identify the type of acid or base (Arrhenius or Brønsted-Lowry) based on its position within the diagram.