Embark on a journey into the realm of atoms and isotopes, where we uncover the fundamental structure of matter and its remarkable applications. Atoms and isotopes worksheet answers provide a gateway to understanding the intricate world of elements, their properties, and their role in shaping our universe.
Delve into the basic structure of atoms, unraveling the interplay between protons, neutrons, and electrons that define their unique identities. Explore the fascinating world of isotopes, where variations in neutron numbers give rise to distinct forms of the same element.
Discover how atomic mass is calculated, unlocking the secrets of isotopic composition and its significance in various fields.
Atomic Structure
An atom is the basic unit of matter and the building block of all elements. It consists of a central nucleus surrounded by electrons.
Nucleus
The nucleus is the central core of an atom, containing protons and neutrons. Protons are positively charged particles, while neutrons have no charge.
Electrons
Electrons are negatively charged particles that orbit the nucleus in specific energy levels. These energy levels are arranged in shells, with each shell holding a maximum number of electrons.
Energy Levels, Atoms and isotopes worksheet answers
Electrons occupy specific energy levels based on their energy. The lowest energy level is closest to the nucleus, and higher energy levels are farther away.
The arrangement of electrons in energy levels determines the chemical properties of an element. Elements with similar electron configurations have similar chemical properties.
Isotopes
Isotopes are variations of the same chemical element that have the same number of protons but different numbers of neutrons in their nuclei. This results in atoms of the same element with different masses. Isotopes are denoted by the element symbol followed by a hyphen and the mass number, which is the total number of protons and neutrons in the nucleus.
For example, carbon-12 and carbon-14 are isotopes of carbon with mass numbers 12 and 14, respectively.
Applications of Isotopes
Isotopes have numerous applications in various fields, including medicine, archaeology, and environmental science.
- Medicine:Isotopes are used in medical imaging techniques such as X-rays, CT scans, and PET scans. Radioactive isotopes are also used in cancer treatment, such as iodine-131 for treating thyroid cancer and cobalt-60 for treating head and neck cancers.
- Archaeology:Isotopes are used in radiocarbon dating, a technique that determines the age of organic materials by measuring the amount of carbon-14 present. Carbon-14 is a radioactive isotope of carbon with a half-life of 5,730 years, making it suitable for dating materials up to 50,000 years old.
- Environmental Science:Isotopes are used to study environmental processes such as water movement and pollution. For example, deuterium, an isotope of hydrogen, is used to trace the flow of water in aquifers and rivers.
Atomic Mass
Atomic mass is the weighted average mass of all the isotopes of an element. It is calculated by multiplying the mass of each isotope by its relative abundance and then adding the products together. The relative abundance of an isotope is the percentage of atoms of that isotope in a naturally occurring sample of the element.
Calculating Atomic Mass
To calculate the atomic mass of an element, follow these steps:
- Obtain the isotopic composition of the element, which includes the mass and relative abundance of each isotope.
- Multiply the mass of each isotope by its relative abundance.
- Add the products from step 2 together.
- The sum obtained in step 3 is the atomic mass of the element.
For example, the atomic mass of chlorine is calculated as follows:
Isotope 1
Mass = 35 amu, Abundance = 75.77%
Isotope 2
Mass = 37 amu, Abundance = 24.23%Atomic mass = (35 amu x 0.7577) + (37 amu x 0.2423) = 35.45 amu
Table of Atomic Masses
The following table shows the atomic masses of some common elements:| Element | Atomic Mass (amu) ||—|—|| Hydrogen | 1.008 || Helium | 4.0026 || Carbon | 12.011 || Nitrogen | 14.007 || Oxygen | 15.999 || Chlorine | 35.45 || Potassium | 39.098 || Calcium | 40.078 || Iron | 55.845 || Lead | 207.2 |
4. Nuclear Chemistry: Atoms And Isotopes Worksheet Answers
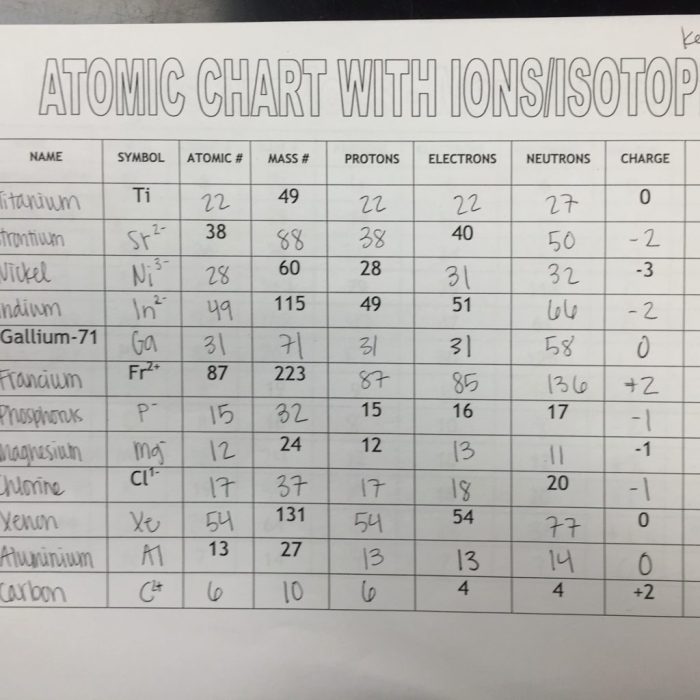
Nuclear chemistry encompasses the study of the structure, properties, and reactions of atomic nuclei. It involves understanding the fundamental forces and interactions within the nucleus, as well as the transformations and applications of nuclear energy.
Nuclear Reactions
Nuclear reactions involve changes in the composition or structure of atomic nuclei. They can be classified into two main types:
- Fission: The splitting of a heavy nucleus into two or more smaller nuclei, releasing a significant amount of energy.
- Fusion: The combination of two or more light nuclei into a heavier nucleus, also releasing energy.
Radioactivity
Radioactivity refers to the spontaneous decay of unstable atomic nuclei, emitting particles or energy to achieve a more stable state. Radioactive isotopes undergo three main types of decay:
- Alpha decay: Emission of an alpha particle (helium nucleus)
- Beta decay: Emission of a beta particle (electron or positron)
- Gamma decay: Emission of gamma rays (high-energy photons)
Nuclear Energy
Nuclear energy is the energy released during nuclear reactions. It is harnessed in:
- Nuclear power plants: Fission reactions are used to generate heat, which is converted into electricity.
- Medical applications: Radioactive isotopes are used in diagnostic imaging (e.g., PET scans) and radiation therapy for treating cancer.
5. Applications of Atoms and Isotopes
Atoms and isotopes play a significant role in various fields, providing valuable insights and practical applications across disciplines.
In medicine, isotopes have revolutionized medical imaging techniques. For instance, radioactive isotopes like Technetium-99m are used in diagnostic scans to visualize and assess the functioning of different organs. Additionally, isotopes like Cobalt-60 are employed in radiation therapy to target and eliminate cancerous cells.
Archaeology
In archaeology, carbon dating, a technique based on the decay of radioactive Carbon-14, has become indispensable for determining the age of ancient artifacts and fossils. By analyzing the remaining Carbon-14 isotopes in organic materials, archaeologists can estimate the time since the organism’s death or the artifact’s creation.
Environmental Science
In environmental science, isotopes serve as valuable tracers to monitor pollution and study climate change. Radioactive isotopes, such as Cesium-137, can be used to trace the movement of pollutants in the environment, helping scientists understand their dispersion and impact on ecosystems.
Stable isotopes, like Deuterium and Oxygen-18, provide insights into past climate conditions by analyzing their variations in ice cores and marine sediments.
Popular Questions
What is an isotope?
An isotope is a variation of an element with the same number of protons but a different number of neutrons, resulting in different atomic masses.
How is atomic mass calculated?
Atomic mass is calculated by multiplying the abundance of each isotope by its mass and summing the products.
What are the applications of nuclear chemistry?
Nuclear chemistry finds applications in power plants, medical treatments, and environmental monitoring, among others.